Hospital Serial Killer – Super Bugs Created With Jumping Genes During Bacterial Sex: Although many of us have heard about killer bugs found in hospitals like MSRA it is not generally appreciated how these bugs develop and spread. Antibiotic resistance genes are able to piggy back onto jumping genes (plasmids or transposons) that can be spread by bacterial sex. Explicit pictures and explanations below…
Most of us are aware of the recent development of killer bugs that have become resistant to many of the antibiotics used against infectious diseases. Our hospitals are favoured breading grounds for these ‘super’ bacteria causing them to thrive in the very places we visit to get treatment against them. Few people however understand how these unpleasant killers become resistant to our drugs. Where does the resistance come from and how can it spread between bacteria? Why do super bugs flourish in hospitals?
Over 40 years ago in 1974 Alan Jacob and Susan Hobbs published research demonstrating that jumping genes are transmitted between bacteria when they engage in sexual activity. Specifically they demonstrated this phenomenon in streptococci bacteria which normally live in the human gut. They belong to a broader group of microbes known as gram positive bacteria – this group causes many of the most unpleasant human diseases. Like humans bacteria store all of their genetic information in chromosomes. Jumping genes can jump out of the chromosome of one bacteria and then transfer into another bacteria as they engage in sex. These jumping genes can then insert themselves into the chromosome of their new host.

At this stage I have to declare a personal relationship with one of the discoverers of antibiotic resistant jumping genes. Less than 5 years after Jacob and Hobbs published their seminal paper I was fortunate enough to carry out a research project under the supervision of Dr. Alan Jacob as part of my first degree dissertation in the Bacteriology Department based in Manchester University’s Medical School. My own research used the same bacterial strains featured in Jacob and Hobb’s original work and I spent the best part of a year trying to better understand this phenomenon of bacterial sex and how it gets hijacked by jumping genes to create the killer bugs that are the scourge of modern hospitals.
So what are these jumping genes? In the scientific community they are normally referred to as plasmids or more recently transposons. In my last article explaining how turning off genes can lead to depression I described a simple way to visualise genes strung along a chromosome like a set of beads on a necklace (the link to my ‘depression’ article is here). Bacteria generally have one very large circular chromosome (‘necklace’) containing all of their genes (the ‘beads’). Each gene contains the construction information for a key piece of equipment needed by a fully functioning bacteria (most of this equipment is in the form of proteins – which are miniature pieces of molecular machinery).

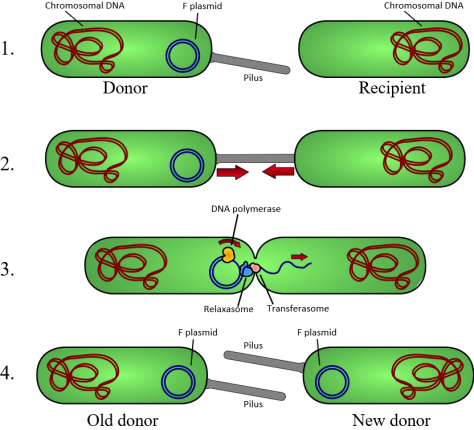
Sometimes bacteria possess genes along a short section of their DNA ‘necklace’ which can cut themselves out of the big chromosome and create a mini necklace – a plasmid (see the diagram above). These genes, that are able to extract themselves out of the main chromosome (necklace), are what we sometimes refer to as jumping genes. They can literally ‘jump’ out of a chromosome and at a later stage re-insert themselves back into the chromosome (or into the chromosome of another bacterium following sex).
Plasmids, with their short strand of genes, can exist either within the much bigger chromosome or they can exist separately from it as a free circle of genetic material floating within the bacterial cell. Although a typical plasmid does not contain as many genes as a whole bacterial chromosome it can carry some very useful genes. For example a plasmid may carry genes which help a bacteria to resist being killed by antibiotics. It was this kind of plasmid that I investigated nearly 40 years ago. It carried resistance to the antibiotics streptomycin, kamaycin, neomycin, erythromycin and tetracycline.

When it comes to survival bacteria can be very versatile. As with higher forms of life they use sexual activities to share useful bits of genetic information with each other. Often bacteria containing plasmids will have small appendages like hairs or tubes on their surface – we can think of these as the ‘male’ bacteria. The photo above is of these appendages or pili on the surface of the streptococcus I worked on in 1979 (sorry about the quality but these electron microscope pics were taken 37 years ago!).
If a hairy male bacterium (containing a plasmid) comes across a ‘female’ bacterium that does not have these pili on its surface they ‘get together’. One of the things I found in my research was the existence of a sex hormone or pheromone given off by the ‘female’ bacteria to entice the ‘males’ into conjugative behaviour. If the conditions are right the ‘male’ will then transfer a copy of his plasmid into the ‘female’ bacterium. In this way any genes on the plasmid such as those for antibiotic resistance can be spread through the local bacterial community (bacteria are not strictly speaking male and female but this analogy should help you to understand what is going on).
OK – this explains somewhat simplistically how bacterial sex works in spreading multiple antibiotic resistance but where did this resistance come from in the first place? To understand this we need to appreciate where most antibiotics exist in nature. Scientists in pharmaceutical companies spend a lot of time messing about with soil. This is because in soil there are huge numbers of different kinds of bacteria and other microbes all living together. This is not however a very peaceful co-existence – the bugs are highly competitive and constantly fire selective killer chemicals into the soil around them to kill off their neighbours. After killing off the competition they can eat their share of the nutrients (in fact they will also eat their dead neighbours).
Pharmaceutical scientists try to find and evaluate these selective killer chemicals produced by microbes. They hope to find ones that can kill disease causing bacteria but will not harm humans. This is how many antibiotics were originally discovered. Some, like penicillin, were simply products made by microbes that very effectively killed other microbes. Others were chemical modifications of microbial products which were more effective or more selective in killing their target organisms.

I produced an article about 4 years ago explaining how bugs living in the soil had also developed defence mechanisms to protect themselves from killer chemicals produced by their neighbours (the link to my soil bacteria article is here). Scientists had discovered that the genes for these antibiotic ‘defence’ mechanisms could be transferred between bugs by – yes you guessed it – bacterial sex. Their results showed that antibiotic resistance found in bugs in hospitals was the same as that found in soil bugs. This meant that hospital bugs may be picking up antibiotic resistance by sex with soil bugs.
In the hospitals we have historically used our armoury of powerful antibiotics in a very uncontrolled and uncoordinated manner. Rather than testing each patient to check which microbial strain causes their disease and then identifying precisely which antibiotic kills that strain they have tended to use antibiotics on a try-it-and-see basis. Here they use the patient as a test laboratory – if the first antibiotic doesn’t work then they try another, and perhaps another until symptoms improve.

In addition to wasting patient time and hospital resources each time an antibiotic is unsuccessfully used it increases the concentration of bugs resistance to that antibiotic. The hospitals are using patients as incubation chambers to select and concentrate antibiotic resistant bacteria. About 90% of the cells in our bodies are not human cells – they are bacterial cells that normally live in harmony with us in our guts and on our skin. Some of these bacteria will be naturally resistant to certain antibacterial agents and through microbial sex with jumping genes they spread the resistance to their neighbours. If we kill all the antibiotic sensitive bugs through uncontrolled antibiotic use the few resistant ones left will have a field day enjoying all the nutrients left behind. Before long most of the bacteria in our bodies will be the drug resistant ones.

Not only are we concentrating antibiotic resistant strains in our hospitals but since we also feed a lot of our farm animals with antibiotics we are also developing antibiotic resistance centres around our farms. Any bugs that happen to get washed out of the farm or hospital into the local soil (perhaps via the sewage) will then be able to transfer their resistance genes through sex with other bacteria in the soil. And so the vicious circle continues – mankind has taken some incredibly useful antibiotic products and effectively made them useless by uncontrolled excessive use in hospitals and by feeding them to farm animals.
The pace of increase in our knowledge about molecular biology is phenomenal and we are already finding that this knowledge is helping us in the fight against terrible diseases like cancer. We should however learn from history – it is over 40 years since jumping genes and bacterial sex were identified as important factors in the spread of multiple antibiotic resistance in hospitals. My own dissertation was written in 1979. Today many of the pathogens identified in our hospitals are resistant to the main drugs we have developed to use against them.
The advance of multiple antibiotic resistance could have been slowed by:
- testing the sensitivity of pathogens isolated from a patient before using drugs – only use antibiotics that work on that pathogen to keep drug usage to a minimum.
- using antibiotics in controlled campaigns – rotating their use instead of using them all at the same time – as soon as the prevalent bugs develop resistance to the current drug you can rotate to a new drug and stop using the first one (until resistance levels in the local bacterial population subsides).
- banning the use of antibiotics similar to those used in human medicine for animal farming purposes.
To see an illustrative video of bacterial sex (parental guidance not necessary) here is a link.
When I prepared the Duggleby family tree going back nearly 1000 years I was struck by how many families were nearly wiped out by diseases like small pox, diphtheria and plague (Link to the tree is here see pages 9, 10, 29, 32, 46, 78, 110 – look for the black boxes). Sadly as we come close to conquering the worst effects of some forms of cancer we may be faced with the return of some of these old diseases due to our inability to apply antibiotics responsibly. Of course we could provide drug companies with financial incentives to develop new antibiotics – welcoming in an era in which new antibiotics will be as expensive as the treatments for leukaemia or AIDS.
Chris Duggleby
Here is a link to the original paper in which Alan Jacob and Susan Hobbs first identified multiple antibiotic resistance plasmid transfer via conjugation in gram positive bacteria (‘jumping genes’). The importance of their work was recently (2016) reconfirmed in an editorial in the Journal of Bacteriology – link here.

If you are interested in reading my other health focused articles try the following
Torture In The Shower – Face and Body Soap Allergies – Main Suspect: Pears Transparent Soap
Toxic Chemicals in Sex Toys – 18 Vibrators, Cock Rings, Love Balls Tested – Only 3 Get All Clear
My T-shirt Made Me Sick – Textile Allergies – Sinusitis From Your Underwear


Well, if anyone can make a depressing, disgusting, and technical subject a fun read, you can. Lots of skill, thought and effort in this article. You are one smart and funny cousin.
Thanks Linda,
your lovely comments brought a ray of sunshine into a rainy, overcast weekend in the German Alps,
Kind regards,
Chris